Symptom Monitoring WIth Feedback Trial
A novel registry-based cluster randomised controlled trial among Australian adults on haemodialysis
Principal Investigators: Rachael L Morton, Stephen McDonald, Chris Brown, Paul Bennett, Fergus Caskey, Cecile Couchoud, Shilpa Jesudason, Andrea Viecelli, Brendan Smyth and William Handke (consumer)
Clinical Project Manager: Lavern Greenham, Trial Coordinator, ANZDATA Registry, SAHMRI,
Trial Operations Cordinator: Portia Westall, NHMRC Clinical Trials Centre, University of Sydney
Trial Onsite Support: Samuel Herzog, Research Assistant, NHMRC CTC,
Alexandra Picorelli, Research Assistant (SWIFT), ANZDATA Registry, SAHMRI
Trial Registration Number: ACTRN12620001061921
Population: Adults aged 18 years and older with ESKD receiving in-centre haemodialysis or haemodiafiltration in Australia
Intervention: Both the EQ-5D-5L (5 questions and Visual Analogue Scale) and IPOS-Renal measures (11 Questions) will be collected by patients using a tablet when they come for their in-centre dialysis sessions
Follow-up: 1 year
Primary outcome: Change in health-related quality of life (measured by validated and widely used EQ-5D-5L questionnaire)
Status: Recruitment Open
Target Recruitment: 2422 participants across 143 centres in Australia and New Zealand

Chief Investigator
Director, Health Economics &
Health Technology Assessment at the
NHMRC Clinical Trials Centre

Clinical Lead
Senior Staff Nephrologist RAH,
Clinical Director of Renal Services for Country Health SA,
Director of Strategy and Policy &
Executive Officer of ANZDATA

Trial Operations Coordinator
NHMRC Clinical Trials Centre

Clinical Trial Coordinator
ANZDATA Registry

Research Assistant
New South Wales

Research Assistant
Queensland
Adults who receive haemodialysis treatment for kidney failure suffer from many problems (symptoms) including tiredness, pain, itching, sleep problems, depression and have reduced quality of life1. About 2 million people worldwide, including 13,000 Australians are treated with haemodialysis. This costs about $1.1 billion per year across Australia. People on haemodialysis also report poor health. Only around 60% of full health. Survival for people who remain on dialysis for 5 years is around 50%, which is lower than all cancers combined2.
SWIFT is the Symptom monitoring WIth Feedback Trial, this trial is being run by Professor Rachael Morton from the University of Sydney and the NHMRC Trials Centre in Sydney and ANZDATA in Adelaide.
An important problem is that standard dialysis care does not focus on outcomes important to patients like quality of life or symptoms; instead the focus is on managing amounts of urea, potassium, phosphate in a patient’s blood. This has led to missed opportunities to help and improve symptom management and overall quality of life. New information from other areas showed symptom monitoring may not only improve quality of life, but also improve overall survival2. SWIFT will aims to show this in a haemodialysis patients.
Table 1. Symptoms in adults with kidney failure
| Symptoms haemodialysis patients experience | Percentage of people with symptom (%) |
|---|---|
| tiredness | 71% |
| itching | 55% |
| constipation | 53% |
| anorexia | 49% |
| pain | 47% |
| sleep problems | 44% |
References:
1 Murtagh FE et al. Adv Chronic Kidney Dis. 200714(1):82-99;
2 Basch E et al. JAMA. 2017;318(2):197-198.
An Australian pilot study was run between September 2019 and March 2020.
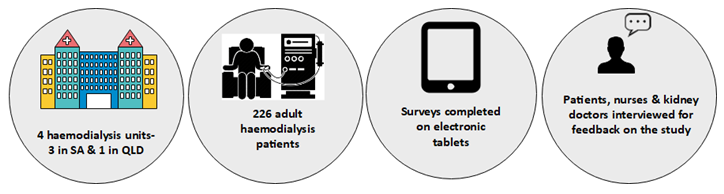
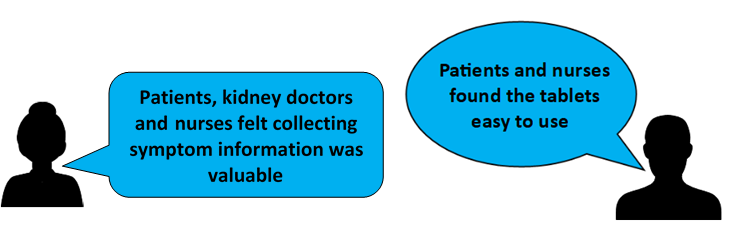
The Pilot study showed that patients and nurses found the tablets easy to use. Patients, nurses and kidney doctors also felt that collecting this information was valuable to the wellbeing of haemodialysis patients. The pilot study also showed that we could run a larger registry trial.
SWIFT wants to know if symptom monitoring with feedback to nurses, kidney doctors and patients, improves health-related quality of life. SWIFT will also test if collecting information about symptoms with a tablet computer is cost effective.
If your haemodialysis unit is involved in SWIFT, they will be in group 1 or group 2. You will be asked to complete a couple of surveys on a tablet computer
Group 1 – Control Arm
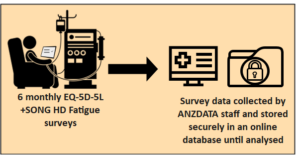
If the haemodialysis unit is in group 1, participants will be asked to complete the EQ-5D-5L and SONG HD Fatigue surveys, every 6 months for one year. These questionnaires take about 5 minutes to complete. At the 12 months, some participants will also be asked for some questions about what they thought about the trial.
Group 2 – Intervention Arm
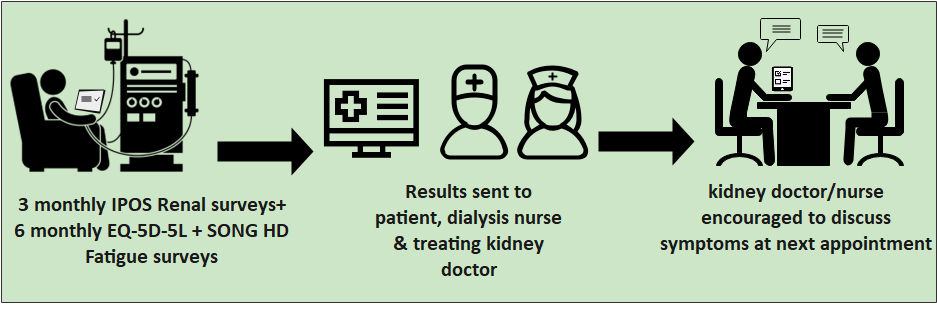
If the haemodialysis unit is in group 2, participants will be asked to complete the IPOS Renal surveys every 3 months and the EQ-5D-5L +SONG HD Fatigue surveys every 6 months. They take about 10 minutes to complete each time. The completed IPOS Renal surveys will be sent to the dialysis nurses and each patient’s kidney doctor who will be asked to follow up symptoms at the next appointment. At the 12 months, some participants will also be asked for some questions about what they thought about the trial.
Target Recruitment
Funding:
- Funding: NHMRC Project grant #1159051
- KHA 2018-RM, NHMRC TRIP Fellowship #1150989
- University of Sydney Robinson Fellowship
SWIFT Newsletters
Video resources
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Contact Us
SWIFT Trial Coordinator, ANZDATA: Dana Alhuziami
Email: swift@anzdata.org.au
Phone: on (08) 8128 4264
Twitter: @SWIFTrial
Ethics approval
Central Adelaide Local Health Network Human Research Ethics Committee
HREC Chair: Mr Ian Tindall
Email: Health.CALHNResearchEthics@sa.gov.au
Phone: +61 8 8222 6841