Targeted Education ApproaCH to Improve Peritoneal Dialysis Outcomes Cluster Randomised Controlled Trial (CRCT)
A pragmatic, multi-centre, multinational, cluster-randomised trial (CRCT), randomising PD units to implement TEACH-PD training modules targeted at PD trainers and incident PD patients versus standard existing practice.

Principal Investigators: Josephine Chow, Neil Boudville
Clinical Project Manager: Laura Robison
Clinical Research Associate: Andrea Valks, Ruth Stanstny
Trial Number: AKTN 17.03
Trial Registration Number: NCT03816111
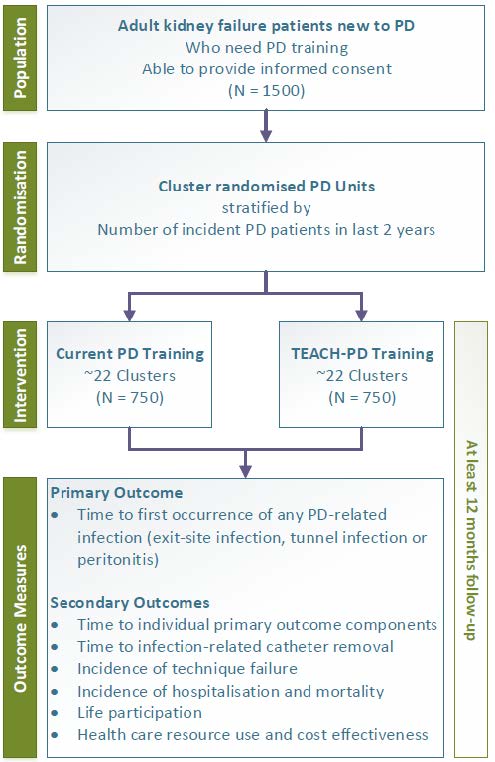
Population: Patients commencing Peritoneal Dialysis for the first time who require training
Intervention: PD training using TEACH-PD training modules
Follow-up: 2 years
Primary outcome: Time to the first occurrence of any PD-related infection (exit site infection, tunnel infection or peritonitis)
Status: Recruitment Open
Target Recruitment: 1500 participants across 44 centres in Australia and New Zealand

University of Western Australia & Sir Charles Gairdner Hospital

South Western Sydney Local Health District
In Australia and New Zealand PD-related infections are the major causes of morbidity and PD technique failure. The burden of PD-related infections, such as recurrent or severe exit site infections and peritonitis, is both high and unacceptably variable between different centres, with the variation appearing to be driven by centre-related factors rather than patient-related factors. Some of the centre-specific disparity in outcomes, including peritonitis rates, has been attributed to minimal attention paid to potentially modifiable centrespecific peritonitis risk factors, including training practices.
TEACH-PD is a registry-based, pragmatic, multi-centre, multinational, clusterrandomised controlled trial (CRCT), randomising PD units to implement TEACH-PD training modules targeted at PD trainers and incident PD patients versus standard existing practices. The modules will be implemented at PD units in Australia and New Zealand to formally evaluate whether, compared with standard care, a standardised training curriculum will reduce the rate of PD-related infections and improve technique survival, resulting in better outcomes for patients receiving PD and significant cost-savings to the community.
To determine whether implementation of standardised training modules based on ISPD guidelines targeting both PD trainers and patients results in a longer time to the composite end-point of exit site infections, tunnel infections and peritonitis in incident PD patients compared with existing training practices.
Inclusion Criteria :
- Patients new to PD;
- Patients > 18 years of age,
- Need to undergo PD training;
- Are able to provide informed consent
Funding
The study has received funding from the BEAT-CKD Program, ISPD, Baxter, QLD Health, Metro South Health Research Support Scheme Research Fund, Medical Council Medical Research Future Fund Rare Cancers, Rare Diseases and Unmet Need Initiative
- The HOME Network
- Australia and New Zealand Dialysis and Transplant Registry
- New Zealand Peritoneal Dialysis Registry



Contact Us
Trial Coordinator, ANZDATA: Kylie Hurst, General Manager
Email: trials@anzdata.org.au
Phone: on (08) 8128 4758
Ethics approval
South Western Sydney Local Health District Human Research Ethics Committee – EC00136
HREC/19/LPOOL/32
HREC Chair: Professor Murray Killingsworth (Chair)
Email: SWSLHD-Ethics@health.nsw.gov.au
Phone: (02) 8738 8304